Internships
Research Development Engineering Intern September 2016 ~ August 2017
About IngMar Medical:

IngMar Medical is the world leader in breathing simulation, with a vision to help raise the quality of patient care through the use of breathing simulators, lung models, and test lungs. The products are used worldwide in development of new respiratory therapy treatments, to test to ensure the quality and safety of respiratory therapy products, and to train clinicians to achieve the highest level of patient care. For further information, click on the logo above for company's website.
My life at IngMar:
During my one-year co-op, I was involved with solving a variety of engineering design challenges with medical devices. By training, I am a mechanical engineer. Working as a R&D engineering intern at IngMar taught me considerable amount of knowledge from real-world applications. Not only was I engaged in tasks within the field of mechanical science, but I also participated in electrical and software projects. Towards the end of my internship, I created a video to reflect upon my experience at the company. It is through this experience that I am able to become more assertive as a young engineer. IngMar plays a critical role in building my career. Click on the draw-my-life image for linkage to its audio file.

- Mucus Dispenser
As a contracted project for C-STARS (Air Force), the goal is to enhance realism in respiratory simulation. During my third co-op rotation, I was delegated the responsibility for developing a device that would simulate
mucus secretion from patient simulators. The requirements were such that this device could be easily controlled by an end user to dispense precise amount, at increments of 5 ml, of highly viscous material (fake mucus made from silicone rubber).
Among challenges that I managed to solve in this project, my most favorite part was designing the control algorithm and the mechanical assembly for this device. The first working prototype that I've built, as illustrated in the video below, was capable
of pushing 30 lbf and accurately detecting the respective stroke positions via an incremental encoder. Using Matlab Simulink and Arduino, I programmed the controls logic with closed-loop control between a pump, a few proportional valves, a micro-switch,
and an incremental encoder from US Digital.
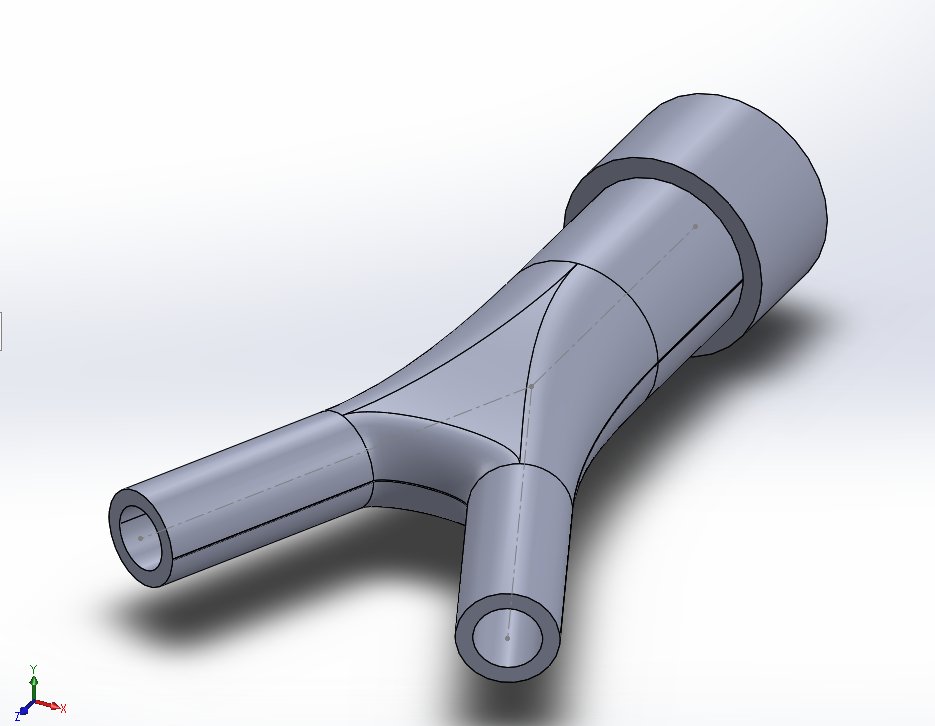
At a high level, the concept was to control air-flow into a piston, and depending on the desired corresponding output in the stroke's distance (entered from a terminal emulator), the air volume would be adjusted by the control algorithm. Figure 1 is a hand-drawn
picture describing the pneumatic system used. Figure 2 through Figure 5 are CAD rendering, as well as pictures taken from other angles of the assembly. Fig 6 shows an early design iteration, the audio of which can be played via this link.
Also, the original audio of the image below for Mucus Dispenser can be played here.
- Chest-rise in Neonate Patient Simulator
While quite much attention has been placed on adult respiratory simulation, the market for neonate patient simulators is increasinly important and has great engineering applications. I took initiative in researching the relevant information, and learned that one great challenge yet to overcome in neonate patient simulation is creating chest rises that would correspond with various physiological states. Unlike adults, neonate patients breathe very subtly, and the respiratory patterns of which would need to be more precisely and carefully designed. To demonstrate my chest-rise concept, I created a control logic between an analog differential pressure sensor, a proportional valve, and a pump to trigger a baby manikin to imitate neonate breathing. Below are an image of a baby manikin, and an animation illustrating controlled chest rise in baby's chest. Click on the chest rise audio for animated illustration.
- Mechanical Conversion Kit
In a collaborative project between IngMar Medical and Laerdal, our goal was to create a mechanical conversion kit so that the end users of both companies can run two patient simulators at once, namely one to create a variety of realistic respiratory patterns, and the other to imitate true physiological responses via a respiratory manikin. The animated files pasted immediately below illustrate the kit at a few design iterations that I made with SolidWorks. For further information about this product, please refer to IngMar Medical's website at this link.
- Airway Pressure Test on False-Postitive Right Main Stem Intubation
In the clinical setting, when a patient is to be ventilated, there's a situation called right main stem intubation, during which a respiratory therapist accidentally intubates a patient in the right main bronchus. Such mistake is sometimes likely to happen because of the inherent geometry of humans' airway. Particularly, since the right main stem is wider and shorter,
and also more vertical than the left main bronchus, if intubation travels far too deep, the tube would easily slide into the right stem. Consequently, air would be occluded from entering the left lung, causing acute respiratory distress.
Laerdal's manikin has a feature to detect when right main stem intubation happens, which is done by monitoring when differential pressure increases past a certain threshold between the two stems. However, when combined with IngMar's lung, this feature could sometimes be tricked and recognized high air pressure from an angled and narrow mechanical pipeline as right main stem. I was assigned to investigae the pressure ranges and flow patterns that would
cause such false-positive interpretations.
I used two piezoresistive type of pressure transducers in my experiments, and Matlab to continuously plot the pressure values as measured. Through TCP/IP, I devised a script such that Laerdal's signal indicating right main stem intubation would be recorded as binary numbers and tracked with respect to time. Meanwhile, this information would be sent to another script monitoring and drawing consequtive pressure points at 75 Hertz, and compared against the
time axis. When the received binary number equals 1, at that instant, it would be collected and ultimately make up a list of recorded times when right main stem was true.
At a high level, as illustrated by Fig. 8, false positive points were marked by pressure transducers between the 13th and 15th second. This timeframe would correspond with the initial stage when air pressure supplied into the patient simulator's airway was starting to build up. Such pattern also repeated in other subsequent experiment trials. Unfortunately, because we weren't able to access some key information from Laerdal regarding the logics that their patient simulator used to interprete pressure variations, we couldn't make official conclusions. The best that could be done at that point was providing Laerdal with this pattern as discovered to help with their analysis.
- Others (Click on Respective Links for Audios)
via Syringe Stroke with Increased Coil Windings
Equipment Engineering Intern June 2014 ~ August 2014
ASE Kaohsiung is ASE's flagship operation, housing the ASE Group's R&D center and operating world-class assembly, wafer bumping and test services. ASE Kaohsiung provides full turnkey services, including substrate design and manufacturing capabilities.
As an equipment engineering intern, one of many responsibilities I had was to simplify and enhance the quality of production by improving designs of machinery components found in IC packaging machines. My experience gave me a considerable depth of understanding in different aspects of product development, from initial concept generation and material selection, to structural integrity validation, and market availability research.
To further elaborate, in one project, I proposed a quick-installation-and-removal mechanism for faster implementation of the heat block, normally found at a deep level within the die-attach machine.
The heat block was made up of wearable material, and assembling it with the machine would require highly complex manipulations. As a result, every calibrations of such machinery component would come with a great cost of time, labor, and price. I suggested several re-design options as a new replacement, among which the most preferable was snap-fitting a protruded opening into a negative space made of rubber,
which could be manually accomplished with a light push. The concept works similarly to an adapter found in the accessory kit for impact screw driver, by which different sizes of screw bits can be easily interchanged.
During my design iterations, I used Creo and Comsol for CAD rendering, CFD and FEA analysis. My goal was to simulate potential mechanical behaviors, and evaluate the subsequent failure risks. One of the concerns, as suggested by the simulation, was that over time the rubber material isolating the system from atmosphere would gradually lose its grip with the interstitial surface near the opening. This was primarily because of the heat expansion/contraction effect from drastically varying temperature required in the manufacturing process.
Taking into consideration that such failure would not actually occur till after the routine calibration point, no further improvements were demanded at that moment. Optimizing air-tightness while minimizing energy required to manipulate an assembly is a great challenge. From this experience, I learned to always be in search of alternatives in the product development process.
Assembly Process Engineering Intern June 2012 ~ August 2012
ASE Kunshan provides semiconductor manufacturing services in IC packaging, design and production of interconnect materials, wafer probing, and electronic manufacturing. This facility offers SO, PDIP, QFN, QFP and BGA packages featuring traditional wire bonding and advanced fine pitch technology.
As an assembly process engineering intern, I learned to trace problems in products from a mass manufacturing line that consists of numerous production stations and machines. When I first started this position, very often I was overwhelmed by the abundance of resources available at my hand. There was a time when a great number of semiconductor products came out with unidentified burn marks, which could only be detected with microscope images, hence a long delay until these defects were discovered by engineers.
I was entrusted with the responsibility to investigate the exact causes of such defects. Unfortunately, not much information could be found regarding the manufacturing history of these products. Meanwhile, it would be a risk to run the next batch of products through the same manufacturing line. We were in an urgent situation, since we couldn’t pause the work que for too long.
My approach to solve this case was by analyzing it concurrently in four directions, where mistakes would most likely take place. Particularly, they were "Human Errors", "Machines", "Materials", and "Methods". After collecting and piecing together all information from these four directions, I transformed them into traceability matrices, and entered these combinations into a statistical program called JMP. With random-pick-and-choose functions, my inputs would be compared against the company’s database recording all the work history of the existing manufacturing stations. By using a self-developed script and this tool, I could derive predictions on a few manufacturing locations that would have the highest probability to fail from the past lessons learned.
I used these predicted results to match accordingly with the current case, which turned out to be quite promising. With this information on hand, a lot of time could be saved from making guesses.
At the end of this project, I familiarized myself well with how industries running various manufacturing facilities managed quality control and kept an accurate track of manufacturing processes.
References for pictures:
1.IngMar Medical. RespiSim System. Digital image. N.p., n.d. Web. https://www.ingmarmed.com/products/respisim-pvi-alternative/.
2.IngMar Medical. IngMar Logo. Digital image. N.p., n.d. Web. https://www.ingmarmed.com/.
3.ASE Group. ASE Group Logo. Digital image. N.p., n.d. Web. http://www.aseglobal.com/en/.